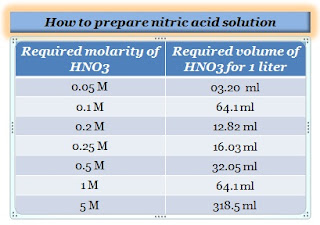
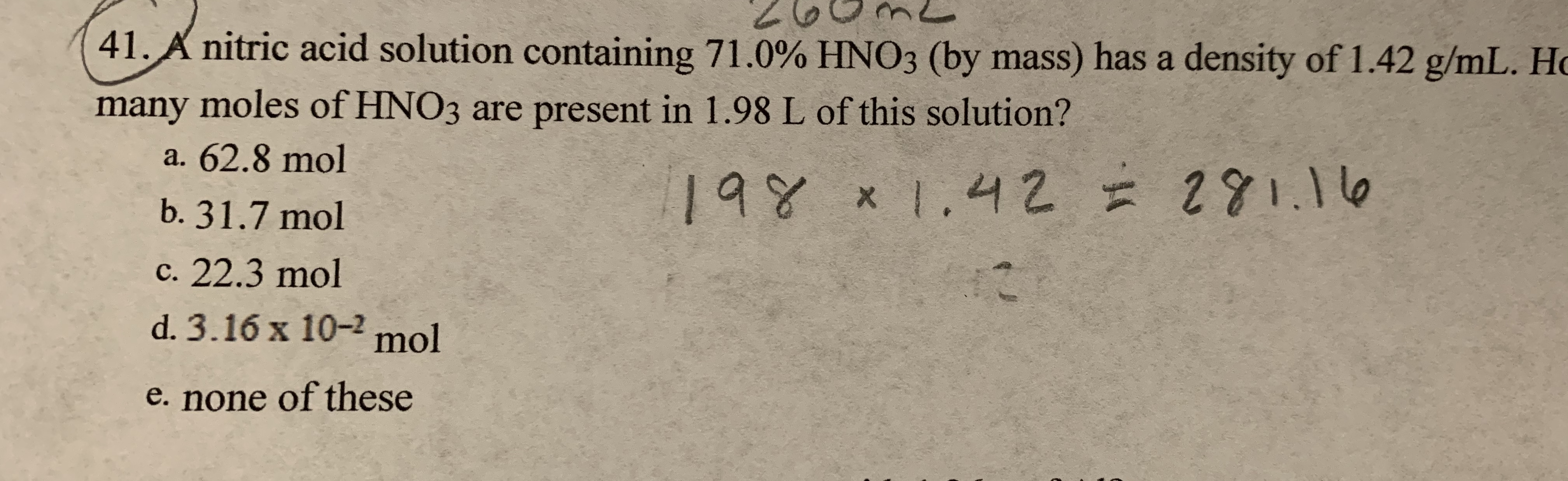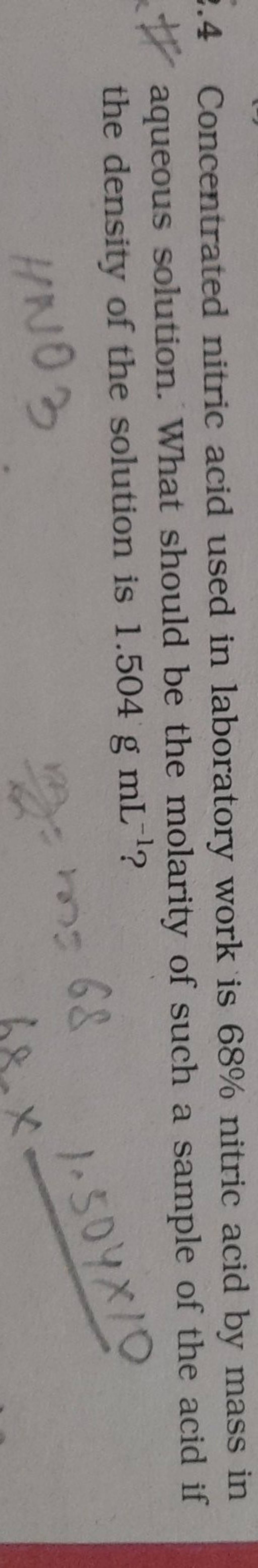PDF) Effects of acid treatment duration and sulfuric acid molarity on purification of multi-walled carbon nanotubes | Abdul Novinrooz and Soghra Mirershadi - Academia.edu

How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO(3) solution ? The concentration of nitric acid is 70 % by mass.

SOLVED: A bottle of concentrated aqueous nitric acid labeled 65% HNOz has density 1.4 g/ml (Mwt =63.01 g/mol) 1What is the molarity of this solution? ILII 214.44 1222 2How many mls of
Nitric acid, 1 l, glass, CAS No. 7697-37-2 | Reagents for Decalcification | Reagents for Histology | Histology/Microscopy | Life Science | Carl Roth - International

SOLVED:A 30.00 % -by-mass solution of nitric acid, HNO3, in water has a density of 1.18 g / cm^3 at 20^∘ C. What is the molarity of HNO3 in this solution?
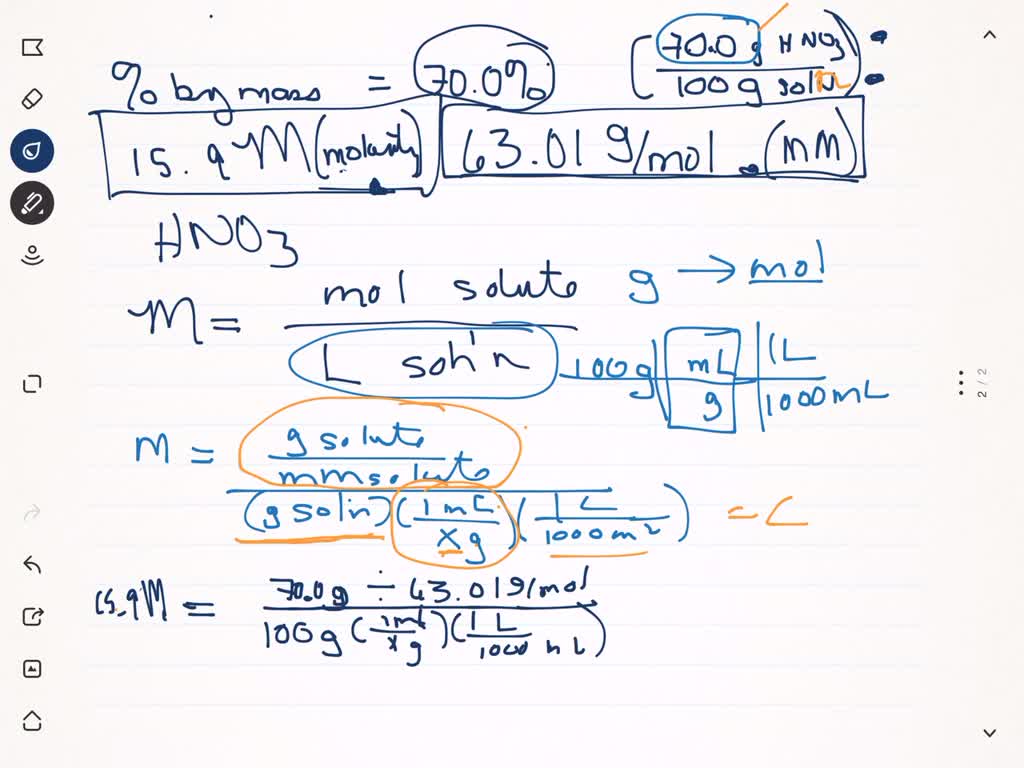
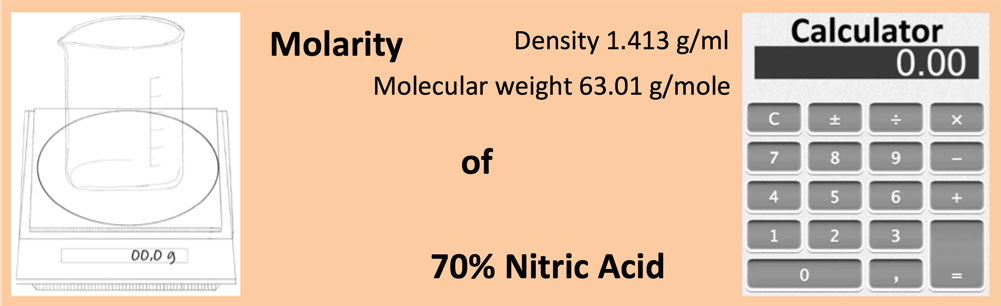
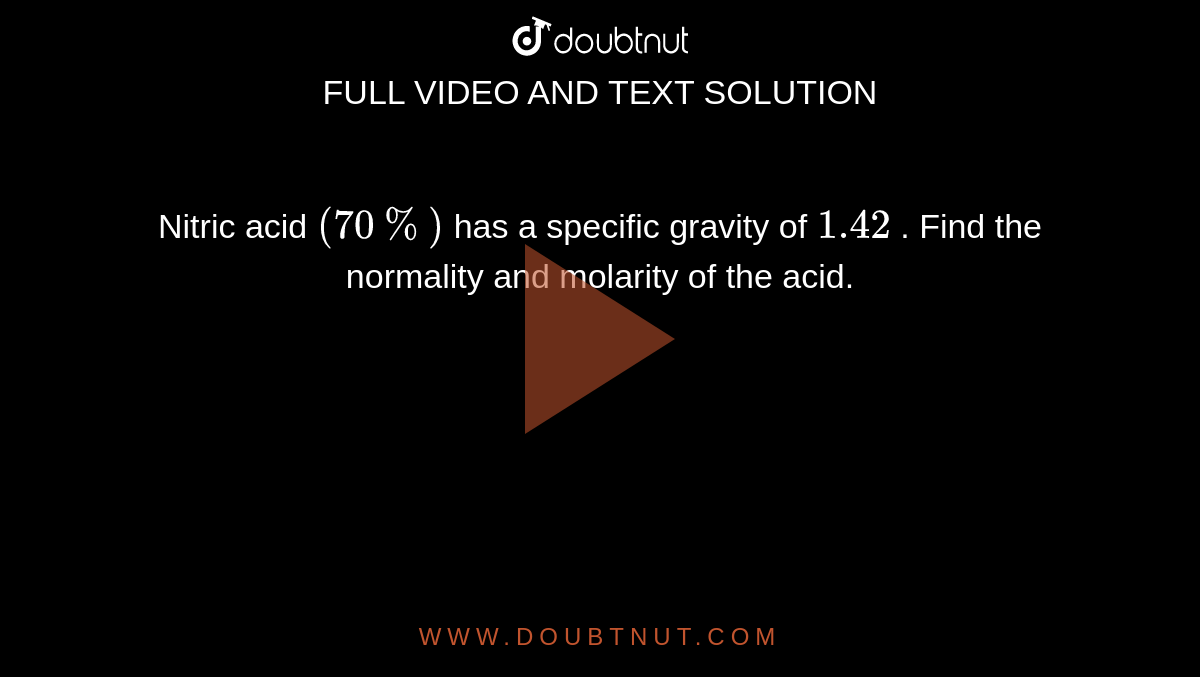
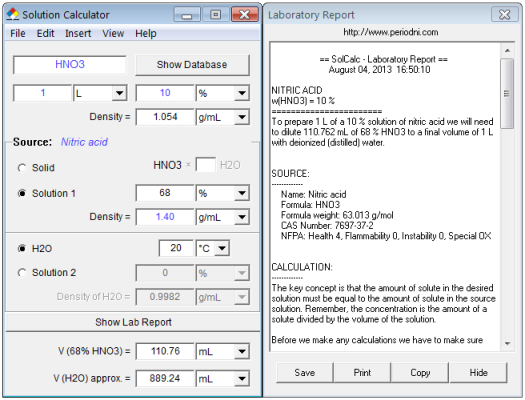
SOLVED:The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15.9 M Calculate the density and the molality of the solution.